Research
Research in our lab spans three areas (detailed descriptions are below):
- From metabolism to movement: quantitative imaging of musculoskeletal and systemic disorders
- Brain health: toxins, relationships, and degeneration
- Computational imaging and machine intelligence
These areas are supported by research grants from the National Institutes of Health, National Science Foundation, National Psoriasis Foundation, and the California Breast Cancer Research Program.
From metabolism to movement: quantitative imaging of musculoskeletal and systemic disorders
Musculoskeletal health monitoring is essential for reducing the global burden of pain, disability, and lost productivity. We adopt an integrative imaging approach to unravel how musculoskeletal disorders, inflammation, and systemic diseases manifest and progress, both in isolated tissues and also across the entire body. By harnessing advanced in vivo imaging modalities, we strive to reveal early, quantitative markers of musculoskeletal dysfunction and recovery, that ultimately will improve function and quality of life. Our projects in this area are below:
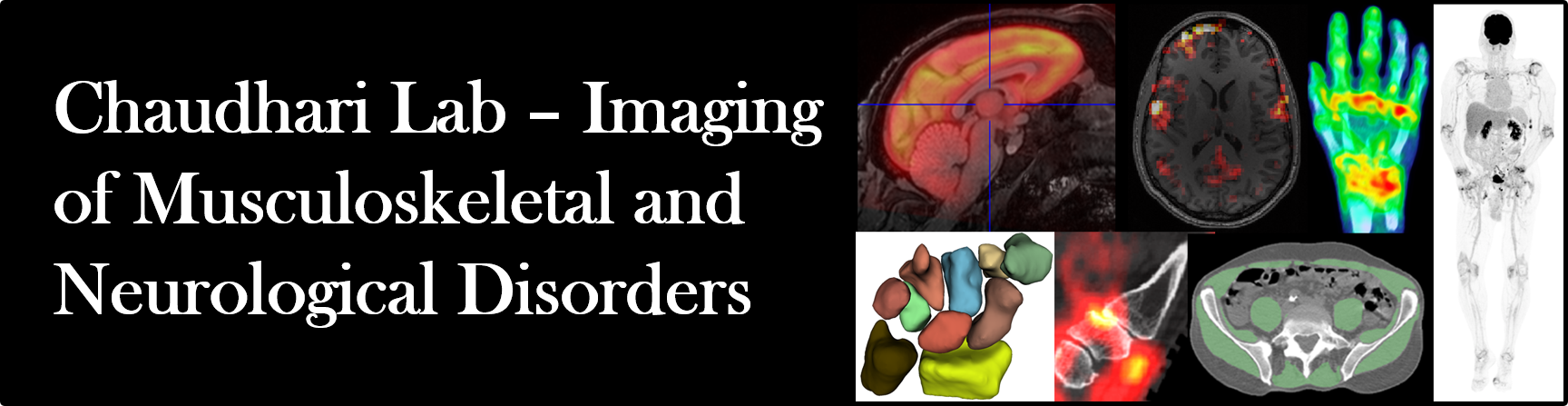
| Autoimmune Arthritis: Autoimmune conditions like psoriatic and rheumatoid arthritis provoke persistent, widespread inflammation, and are known to trigger an intricate cascade of biological events, impacting not only the joints but muscles, bones, ligaments and organs throughout the body. Tools to quantitatively measures systemic immune activity are currently lacking. Using total-body PET/CT and whole-body MRI, our lab explores how chronic immune activity impacts the entire body, including the musculoskeletal, neurological, cardiovascular and hepatic systems. Through these studies, our goal is to provide clinicians with comprehensive, molecular and anatomic maps of effects of these debilitating diseases, in turn, guiding diagnosis and treatment selection and monitoring. Key references: Chaudhari et al., Br J Radiol 2016; Abdelhafez et al., J Nucl Med 2022; Raychaudhuri et al., Rheumatology 2025. |
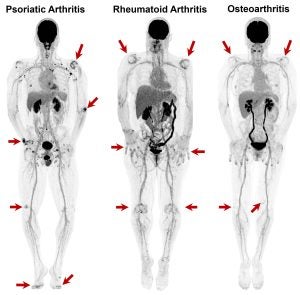 |
| Myofascial Pain: Myofascial pain is a multifactorial disorder characterized by chronic, regionally localized or systemic muscle pain and dysfunction, for which objective biomarkers have historically been lacking. Our research aims to address this gap by developing and validating novel PET/CT-based biomarkers that can detect and quantify biochemical changes associated with myofascial pain within muscle and connective tissue. These methods aim to enable precise mapping of tissue metabolism, perfusion, and inflammatory activity, therefore, rigorous characterization of myofascial tissue pathology, facilitate longitudinal assessment of therapeutic efficacy, and provide a foundation for mechanistic studies that advance the precision and personalization of chronic pain management. Key references: Mazza et al., Am J Phys Med Rehabil 2021; Abdelhafez et al., SNMMI 2025. |
 |
| Joint Movement and Biomechanics: The complexity of joint movement, encompassing translational and rotational degrees of freedom, load transmission, and tissue deformation, calls for imaging modalities that can faithfully reconstruct motion as it occurs. Our lab pioneered the use of advanced real-time MRI techniques with high temporal resolution, reaching up to 78 frames per second, to visualize joint kinematics and biomechanics in vivo. We have demonstrated the ability to detect and quantify subclinical instabilities and aberrant loading patterns that may precede overt injury or degeneration. These innovations inform individualized therapeutic strategies, and enable rigorous evaluation of surgical and rehabilitative outcomes. Key references: Boutin et al., PLoS One 2013; Shaw et al., PLoS One 2019; Chaudhari et al., Br J Radiol 2023 |
Real-time MRI of the moving wrist at 78 frames per second
|
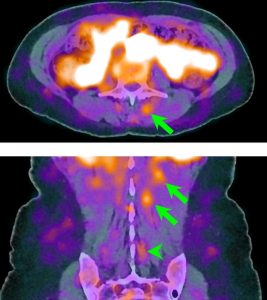
| Frailty: Sarcopenia, marked by progressive skeletal muscle loss, can undermine physical capacity and resilience, increasing frailty and health risks. Our lab’s studies have advanced imaging approaches to map and quantify muscle quality across the entire body. Notably, we have defined optimal methods for measuring muscle characteristics, challenged conventional measures such as body mass index, and demonstrated that our refined methodologies improve the prediction of adverse outcomes. This research is aimed at offering clinicians highly accurate, opportunistic tools for evaluating muscle health, supporting early detection and guiding targeted interventions for improved strength and independence. Key references: Foster et al., J Nat Sci 2018; Zhou et al., Ann Nucl Med 2019; Chaudhari et al., PET Clin 2022. |
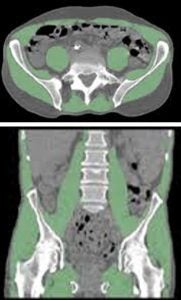 |
Brain health: toxins, relationships, and degeneration
The human brain is a dynamic organ, constantly translating genetic and biochemical signals into responses shaped by life’s environment and the complexities of social interaction.We pursue a multidimensional approach towards understanding brain health, investigating how environmental toxins, social dynamics, and degenerative diseases leave their signatures on neural circuits long before symptoms surface. Using advanced neuroimaging, we aim to provide in vivo measures for early detection, prevention, and precise intervention, helping to safeguard cognition and emotional well-being.
| Brain’s Response to Neurotoxins: We develop and apply advanced PET and MRI techniques to unravel how acute exposure to chemical threat agents initiates neuroinflammation, blood-brain barrier disruption, and changes in brain connectivity as part of the UC Davis CounterACT Center of Excellence. We also investigate the neurobiological impacts of other environmental exposures, such as air pollution, employing state-of-the-art neuroimaging methods. These methodological innovations allow us to detect subtle, early brain responses to a range of toxins, often before clinical symptoms manifest. The objectives of our research are to provide sensitive biomarkers to understand underlying mechanisms, identify at-risk populations, guide timely intervention, and inform public health strategies to safeguard neurological health. Key references: Bernardino et al., Neurobiol Dis 2023; Andrew et al., J Neuroinflammation 2024; D’Almeida AJ, Neuropharmacology 2024. |
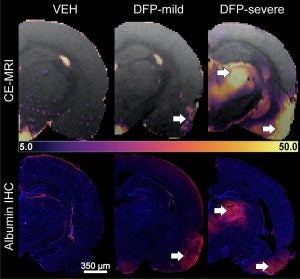 |
| Neurobiology of Social Bonding: Social behaviors emerge from the interplay of complex neural circuits and neurochemical systems that have evolved to support bonding, affiliation, and emotional resilience. By leveraging advanced neuroimaging in collaboration with the laboratory of Dr Karen Bales, our research traces how experiences of attachment and isolation modify the activity of brain regions critical for social cognition and regulation. Mapping these dynamic networks could provide information about how individual differences in social experience can shape vulnerability or resilience to mood disorders, and opens new pathways for understanding, preventing, and treating conditions like anxiety and depression. Key references: Zablocki-Thomas et al., Horm Behav 2023; D’Almeida et al., Molecular Imaging 2025. |
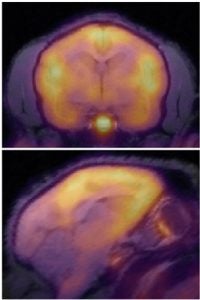 |
| Alzheimer’s Disease: Alzheimer’s disease and other dementias originate years before clinical symptoms emerge, as molecular and structural changes silently unfold in the brain. In collaboration with Dr Pamela Lein’s lab and Dr John Morrison’s lab we deploy advanced neuroimaging biomarkers to detect early synaptic loss, abnormal protein aggregation, and neuroinflammatory processes that are hypothesized to drive cognitive decline. By identifying these early but central changes with in vivo imaging techniques, this work offers the promise to refine risk prediction and a platform for preclinical intervention studies to identify therapeutic approaches. Key references: Beckman et al., Alzheimer’s & Dementia. 2024; Taha et al., JCI Insights, 2025. |
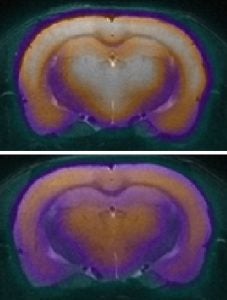 |
Computational imaging and machine intelligence
Advances in medical imaging technology have revolutionized our understanding of biological structure and disease, yet decoding the complexity within medical images demands rigorous, quantitative frameworks. By synergizing mathematical modeling, statistics, and machine intelligence with biomedical imaging, we aim to enable new paradigms in understanding anatomy and pathophysiology, and in patient care.
| Computational Anatomy: Understanding the intricate shapes, sizes, and relationships between bodily structures is central to diagnosing and treating disease. Through mathematical techniques such as diffeomorphic mapping, statistical shape analysis, and multi-organ atlas construction, our lab generates high-dimensional digital models that capture the diversity of brain, bone, muscle, and organ morphology across populations. These approaches facilitate phenotyping based on structure-function relationships, objective comparison between healthy and diseased tissues, and support prediction of outcomes for interventions. The models also provide a foundational framework for testing mechanistic hypotheses, understanding developmental and degenerative processes, and informing healthcare personalized approaches. Key references: Chaudhari et al., Phys Med Biol 2014; Joshi et al., IEEE Trans Med Imaging 2016; Foster et al., J Biomechanics 2020. |
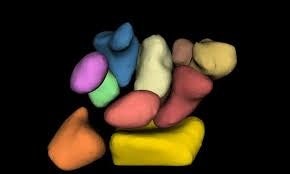 |
| Machine Learning: Extracting quantifiable imaging-based insights from large, multidimensional imaging datasets necessitates the use of artificial intelligence algorithms capable of automating complex analysis tasks. Our lab develops and applies deep learning architectures to segment anatomical structures, detect pathological features, and precisely quantify changes in tissue composition or signal intensity. These methods are validated for consistency, scalability, and sensitivity to subtle variation, reducing observer-dependent error and facilitating both cohort-level research and real-time, individualized assessment. Integration of AI-driven segmentation with computational anatomy is able to accelerates hypothesis testing, enabling population-level studies of disease pathology, and expanding the prospects for reproducible, quantitative precision medicine. Key references: Foster et al, Comput Med Imaging Graph 2019; Porter et al, J Neurosci Methods 2024; Stamos et al, Am J Biol Anthropol 2024; Porter et al, Comput Biol Med 2025 |
 |